Drug-target interaction (DTI) prediction has become a foundational task in drug repositioning, polypharmacology, drug discovery, as well as drug resistance and side-effect prediction. DTI identification using machine learning is gaining popularity in these research areas. Through the years, numerous deep learning methods have been proposed for DTI prediction.
Nevertheless, prediction accuracy and efficiency remain as key challenges. Pharmaco-electroencephalogram (pharmaco-EEG) is considered valuable in the development of central nervous system-active drugs. Quantitative EEG analysis demonstrates high reliability in studying the effects of drugs on the brain. Earlier preclinical pharmaco-EEG studies showed that different types of drugs can be classified according to their mechanism of action on neural activity. Here, we propose a convolutional neural network for EEG-mediated DTI prediction. This new approach can explain the mechanisms underlying complicated drug actions, as it allows the identification of similarities in the mechanisms of action and effects of psychotropic drugs.
- Project name: ANN4EEG
- GitHub repository: https://github.com/neuroeeg/ANN4EEG
- Implementation: https://cmi.to/r2/ (in Russian)
- i-EEG data: dataset_set_1000.zip (274.7 Мб).
- Operating system (s): Platform independent.
- Programming language: Python.
- License: GNU GPL v3.
Research description
Title of the project: A New Transdisciplinary Machine Learning-based Approach for Drug-target Interaction Prediction and CNS-active Drug Discovery
Keywords: drug-target interaction prediction, drug discovery, EEG, patch clamp, signal classification, deep learning, neural networks
Goals and tasks of the project
Research and development of a new transdisciplinary machine learning-based approach for drug-target interaction prediction and CNS-active drug discovery
- Neural activity recording across multiple levels of brain organization after drug administration/application (to generate a training dataset of compounds with a known mechanism and therapeutic value)
- intracranial EEG (i-EEG)
- local field potential (LFP)
- multiunit activity (MUA)
- single unit activity (SUA)
- patch clamp
- Development (involving iterative structural-parametric optimization) and training of machine learning algorithms for signal analysis
- convolutional neural networks (CNN) based algorithms
- recurrent neural network (RNN) based algorithms
- other signal analysis methods
- Prediction of the pharmacological effects and mechanisms of action of new compounds
- Verification (using classical pharmacological methods to validate the predicted effects
and mechanisms of action)
Project description
The high rates of failure in central nervous system (CNS)-active drug discovery, in particular of the first-in-class therapeutics with new modes of action, highlights a clear unmet need to improve the success rate in psychotropic drug discovery [1]. Drug-target interaction (DTI) prediction has become a foundational task in drug repositioning, polypharmacology, drug discovery, as well as drug resistance and side-effect prediction [2].
At present, the ligand-based, target-based, and chemogenomic methods are recognized as three key computational approaches for predicting DTI [3]. Classic DTI prediction approaches are often ineffective; for example, in cases of multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease, as well as other neurodegenerative and psychiatric diseases. This is because (a) an insufficient number of ligands are synthesized or not synthesized at all or (b) adequate targets are not identified [3].
The specificity of psychotropic drug action is reflected in the bioelectric activity of the brain. By binding to targets with complementary structures, psychotropic drug molecules modify the electrical behavior of neurons and lead to specific changes in brain signals. These biological reactions correlate with target binding and can be analyzed using computational models. This concept is supported by abundant evidence from several previous pharmaco-EEG studies [4]. Pharmaco-EEG is very important in the development of CNS-active compounds, as well as drug resistance and side-effect prediction. The modulation of serotonergic, dopaminergic, noradrenergic, cholinergic, or opioidergic neurotransmission causes specific changes in EEG frequency [5]. Many electrophysiological signal features are considered characteristic and unique to specific compounds [6].
Modern machine learning methods help extract information from neural activity signals (i-EEG, LFP, MUA, SUA) and are considered cutting-edge neuroscientific tools [7].
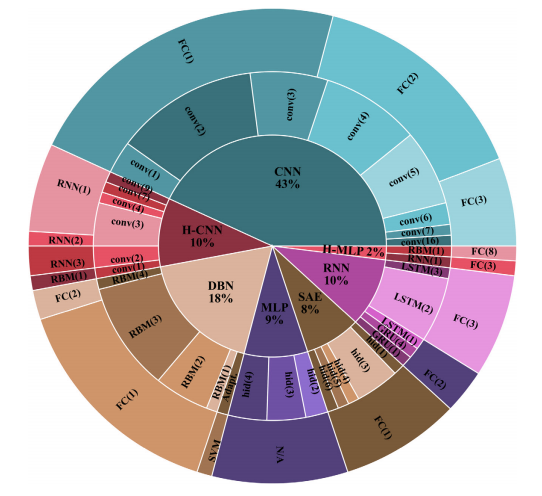
Figure 1. Deep learning architectures across all EEG studies. The inner circle shows the general deep learning strategy, the middle circle shows the primary design feature for the specified deep learning strategy, and the outer circle shows the secondary design characteristic. Key—Adapt.: adaptive number of RBM’s conv(#): number of convolutional layers, CNN: convolutional neural network, DBN: deep belief network, FC(#): number of fully connected layers, hid(#): number of hidden layers, H-CNN: hybrid CNN, H-MLP: hybrid MLP, MLP: multi-layer perceptron, RBM(#): number of restricted Boltzmann machines, RNN: recurrent neural network, RNN(#): number of recurrent layers, SAE: stacked auto-encoders.
In a preliminary study, we have shown that even in the absence of ligands with the same mechanism of action in the intracranial EEG-signals dataset (DOI: 10.17632/gmkbhj28jh.1), our deep learning-based algorithm (Figure 2) could predict the possible effects of a substance, which opens up vast opportunities for the research and development of new anticonvulsants or other substances with psychotropic activity. In addition, there is a high potential for using this approach for repositioning drugs that are already used in clinical practice.
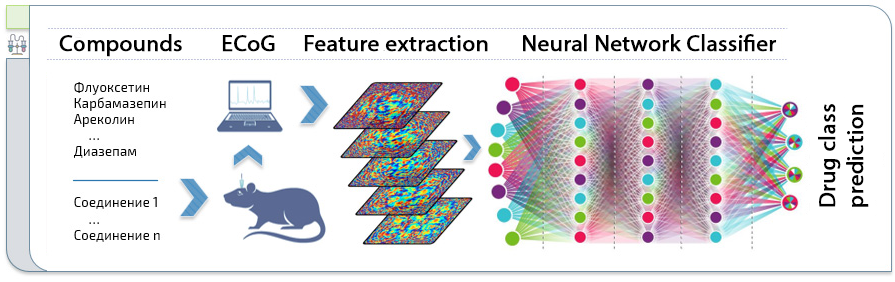
Figure 2. Overall study design. Electrocorticogram (ECoG) is recorded from the animal after drug administration and fed into the neural network (an MLP in this case). The outputs of the last layer are clustered and then the distances between the clusters are calculated.
Expected results
a) development of a novel transdisciplinary approach for drug-target interaction prediction
b) discovery of new CNS-active substances
c) understanding of new fundamental organizational principles of the brain
Research infrastructure available for the project
The laboratory, provided with a healthy workplace environment and working conditions, contains modern high-tech equipment (Figure 3) such as:
- patch clamp setups
- cerebral multi-channel microelectrodes and data acquisition system
- neurosurgical stereotaxic instruments
- low-noise laboratory electroencephalograph
- programmable pulse generator
- automated behavioral and cognitive abilities testing equipment
- supercomputer with 32 TFLOPS of computing power
Figure 3. Scientific equipment available at the laboratory. The equipment, in order from left to right starting on the first row, are microprobe (NeuroNexus), MEA, patch clamp setup, programmable pulse generator (A.M.P.I.), data acquisition system (Biopac), supercomputer (T-platforms).
Close scientific collaboration with chemical laboratories ensure the availability of new substances for research. Full-service vivarium facilities are also available.
References:
[1] Danon, Jonathan J., Tristan A. Reekie, and Michael Kassiou. “Challenges and opportunities in central nervous system drug
discovery.” Trends in Chemistry 1, no. 6 (2019): 612-624.
[2] Bagherian, Maryam, Elyas Sabeti, Kai Wang, Maureen A. Sartor, Zaneta Nikolovska-Coleska, and Kayvan Najarian. “Machine
learning approaches and databases for prediction of drug–target interaction: a survey paper.” Briefings in bioinformatics (2020).
[3] Li, Li, Ching Chiek Koh, Daniel Reker, J. B. Brown, Haishuai Wang, Nicholas Keone Lee, Hien-haw Liow et al. “Predicting
protein-ligand interactions based on bow-pharmacological space and Bayesian additive regression trees.” Scientific reports 9, no. 1
(2019): 1-12.
[4] Brienza, Marianna, Patrizia Pulitano, and Oriano Mecarelli. “Effects on EEG of Drugs and Toxic Substances.” In Clinical
Electroencephalography, pp. 715-729. Springer, Cham, 2019.
[5] Drinkenburg, Wilhelmus HIM, Gé SF Ruigt, and Abdallah Ahnaou. “Pharmaco-EEG studies in animals: an overview of
contemporary translational applications.” Neuropsychobiology 72, no. 3-4 (2015): 151-164.
[6] Nahmias, David O., Eugene F. Civillico, and Kimberly L. Kontson. “Deep learning and feature based medication classifications
from EEG in a large clinical data set.” Scientific Reports 10, no. 1 (2020): 1-11.
[7] Craik, Alexander, Yongtian He, and Jose L. Contreras-Vidal. “Deep learning for electroencephalogram (EEG) classification tasks:
a review.” Journal of neural engineering 16, no. 3 (2019): 031001.